Emulsions as Reaction Volumes for Nanoparticle Production
An emulsion is the fine dispersion (mixing) of two immiscible liquids, e.g. water and oil. The one liquid is distributed as droplets throughout the other liquid. If no separation energy (for example, from stirring) is introduced, the two liquids tend to separate. The droplets of the dispersed liquid coalesce (flow together) and the two liquid phases separate into two layers due to the difference in density. In order to prevent separation, the emulsion must be stabilized with so-called emulsifiers. The emulsifiers consist of a hydrophilic and a hydrophobic end and situate themselves between the two liquids at the so-called phase boundary layer, preventing coalescence and thus a separation of the two liquid phases. Known emulsions are milk, mayonnaise, salad dressing and creams, for example.
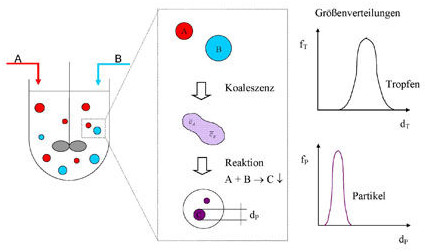
Emulsions are, however, also a frequently used reaction medium in the chemical industry, since dispersing one phase into fine droplets creates a large surface area for exchange processes. However, it is also possible to regard the emulsion droplets themselves as a (micro) reaction volume in which chemical reactions can be carried out. For example, fine particles, so-called nanoparticles with defined characteristics, can be precipitated. These are increasingly being used in modern industry (e.g., fine magnetic particles for high-performance data carriers). In order to be able to carry out the reaction in a controlled manner, the properties of the emulsion must be known. In addition to the physical properties of the pure substances used, the droplet size distribution, the composition of the emulsion, and the exchange rates between the droplets are of particular interest.





